Marine Lines, Mumbai, Maharashtra
- GST NO. : 27AABPR4112P1ZH
| Business Type | Manufacturer, Exporter, Supplier, Retailer, Wholesaler, Trader, Distributor, Importer, Buying House |
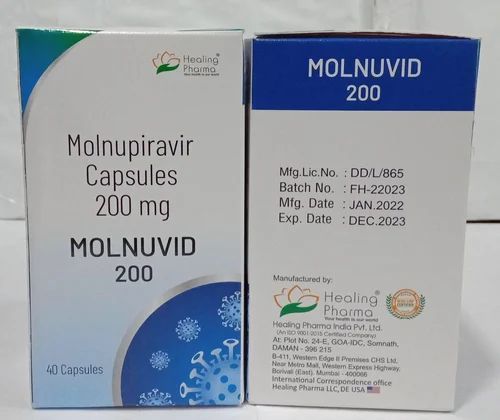
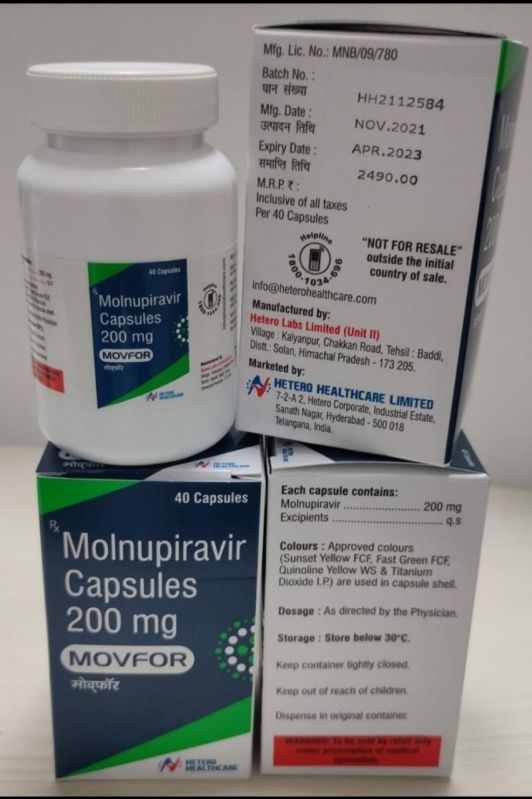
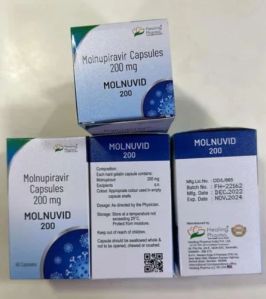
| Brand Name | Movfor |
| Color | White |
| Form | Capsules |
| Click to view more | |
Preferred Buyer From
| Location | Worldwide |
Product Details
Product Description
Molnupiravir (development codes MK-4482 and EIDD-2801) is an experimental antiviral drug which is orally active and was developed for the treatment of influenza. It is a prodrug of the synthetic nucleoside derivative N4-hydroxycytidine, and exerts its antiviral action through introduction of copying errors during viral RNA replication.
The drug was developed at Emory University by the university's drug innovation company, Drug Innovation Ventures at Emory (DRIVE). It was then acquired by Miami-based company Ridgeback Biotherapeutics, who later partnered with Merck & Co. to develop the drug further.
Safety controversyIn April 2020, a whistleblower complaint by former Head of US Biomedical Advanced Research and Development Authority (BARDA) Rick Bright revealed concerns over providing funding for the further development of molnupiravir due to similar drugs having mutagenic (DNA damaging) properties.[3] A previous company, Pharmasset, that had investigated the drug's active ingredient had abandoned it. These claims were denied by George Painter, CEO of DRIVE, noting that toxicity studies on molnupiravir had been carried out and data provided to regulators in the US and UK, who permitted safety studies in humans to move forward in the spring of 2020. Also at this time, DRIVE and Ridgeback Biotherapeutics stated they planned future safety studies in
Looking for "molnupiravir capsules" ?
Explore More Products